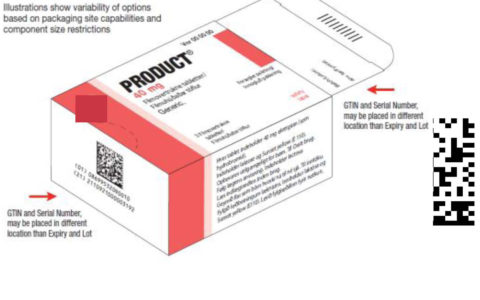
Serialisation
Issues on the introduction of required safety features for medicines Article 54 of Directive 2001/83/EC of the European Parliament and of the Council of 6 november 2001 on the Community code relating to medicinal products for human use specifies that medicinal products subject to prescription shall bear the safety features that enable the authenticity of

